
Each shell is represented by a number, ′ n ′, which is known as a shell number or energy level index.
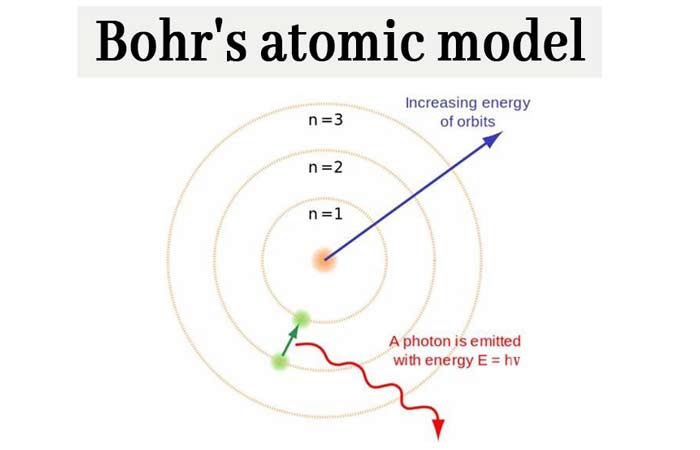
Electrons in different shells have different energies. The frequency of the emitted photon is then given byĮlectron distribution in orbit or shells According to atomic models, electrons move around the nucleus of atom in electron shells. When it does so, a photon is emitted having energy equal to the energy difference between the initial and final states. Bohrs third postulate incorporated into atomic theory the early quantum concepts that had been developed by Planck and Einstein.It states that an electron might make a transition from one of its specified non-radiating orbits to another of lower energy.Thus the angular momentum (L) of the orbiting electron is quantised. This postulate states that the electron revolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of h/2 where h is the Plancks constant ( = 6. Bohrs second postulate defines these stable orbits.

These are called the stationary states of the atom. According to this postulate, each atom has certain definite stable states in which it can exist, and each possible state has definite total energy.

The Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity.


 0 kommentar(er)
0 kommentar(er)
